|
||
 |
||
|
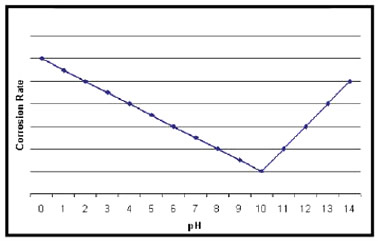
Key Factors in Corrosion and Metalworking Fluids 1. Every metalworking fluid formula has a "sweet spot" where the concentration should be maintained. Checking the concentration frequently will prevent performance and environmental problems. Heat removal is one of the most important functions of a metalworking fluid. Effective heat removal yields good tool life and dimensional accuracy of parts. Water has a greater capacity for removing heat than oil; however, water alone in contact with freshly machined metal leads to corrosion. Thus, corrosion is a problem faced by every user—and manufacturer—of water diluted metalworking fluids. 2. Seasonal Corrosion. Corrosion can occur at any time during the year, but normally it occurs more often during summer months, when temperatures and the relative humidity are high. For example, in the USA corrosion is not as prevelant in arid states, such as Colorado, New Mexico, Arizona, Utah and California, because the relative humidity is usually low. As temperature increases, the rate of all chemical reactions also increases. This includes corrosion. High temperature, in the presence of moisture and oxygen in the atmosphere, is the reason corrosion occurs more during the summer months. 3. Moisture condenses on a metal part and acts as an electrolyte to form a galvanic cell. Many rust preventive liquids produce decent protection in cool months, but fail in the hottest months of the year. On the other hand, increasing the concentration of the RP liquid can create problems in the central system mix such as foam and potential skin irritation problems. 4. pH. The pH of a metalworking fluid is a factor in controlling corrosion. A high pH, between 9 and 10, will protect ferrous metals but will adversely affect the corrosion control of non-ferrous metals, such as aluminum, brass and bronze. When the pH is low in an individual machine, the easiest solution to the problem is to dump, clean and recharge with a new mix of the fluid product at the recommended concentration. If treating a central system mix, which is being used on ferrous metals, adjust the pH to between 8.8 and 9.2 with the proper additives. A mix with an excessively high pH is usually a sign of contamination, and the mix should be dumped and recharged. If non-ferrous metals are being machined and staining or pitting is a problem, check the product information to determine if the product was designed for use with non-ferrous metals. pH measurement is the watchdog; it is critical to control the pH value of the metalworking fluids to within a narrow band. The graph below shows that the lowest rates of corrosion occur at around pH10, with corrosion effects increasing if the pH value of the water becomes acid or alkali. Corrosion and Metalworking Fluids Waters that contain more than 100 ppm chloride, more than If aggressive water is suspected, take a sample of the water and have it completely analyzed. When a central system fluid is suspected to cause corrosion, submit a sample of the mix for ion determination. If the chloride, sulfate or nitrate content is higher than the acceptable limits, the user may have to change sources of water. It may become necessary, for example, to change to a blend of deionized or distilled water with the regular water. A change to another metalworking fluid with superior corrosion control may also be an answer. Many desirable properties of metalworking fluids literally can be destroyed by chemical reaction with dissolved water solids. The most familiar example of this is the effect of “water hardness,” which is essentially the calcium and magnesium content of the water. The divalent ions react with soaps, wetting agents and emulsifiers to form compounds with limited solubility. The formation of these insoluble compounds depletes rust inhibitors, which results in rusty parts and machines. Hard water is any water containing greater than 250 ppm calcium carbonate or 15 ‘grains’ hardness. The higher the hardness is, the more prone the fluid is to corrosion. Conductivity is another means of determining the quantity of dissolved ions in the mix. Higher conductance promotes corrosion, mix instability, residue and other problems. Conductivities of greater than 4 Millisiemens per cm are considered high. 6. Bacteria. High bacteria counts in the fluid mix can lead to corrosion. Bacteria consume metalworking fluid components, and the by-products of this activity can lower the mix pH. The metabolic products If an individual machine’s mix has a high bacteria count, the easiest solution to the problem is to dump the old fluid, clean the sump with a good machine cleaner, rinse with fresh water, and then recharge with a new fluid mix at the recommended concentration. If it is impractical to dump the charge, treat with an appropriate microbiocide. In a central system, preventive measures including the correct use of microbiocides to prevent and control bacterial growth are recommended. 7. Lean Concentration. Ingredients in a metalworking fluid are designed to be effective within a specified dilution range. If the fluid becomes leaner than the specified range, the leaned out ingredients may not be able to perform their designed jobs. This also applies to the rust inhibitors, which may not be able to protect the newly ground or machined parts from corrosion. Check fluid concentration frequently and routinely. Titration methods and refractometers are the best methods of concentration determination. Add concentrate to obtain the recommended dilution. In individual machines, do not attempt to balance the fluid with the use of additives. Dump and recharge. 8. Broken Emulsion. Rust can happen if an emulsion breaks, in which case the instability of the emulsion will make the rust inhibitors ineffective. If the emulsion appears watery and the sample shows stratification, the emulsion may be unstable or broken. To solve this problem in individual machines, the best solution would be to dump, clean the machine, rinse with fresh water, and recharge with a metalworking fluid mix at the recommended concentration. Broken and unstable emulsions are often caused by hard water or excessive bacterial contamination. It is important to determine the cause of the broken emulsion before taking corrective action. When dealing with a central system, it may be possible to use an additive that is an emulsifier to re-emulsify the product. If time permits, send samples to the fluid supplier for tests. If time does not permit, then run jar tests on site to determine what additives to use and the proposed dilution ratio. 9. Dirt Recirculation. Small metal particles in a metalworking fluid are sometimes referred to as "dirt" or "swarf." Swarf deposited on the part and not properly washed away forms a galvanic cell and rust will occur underneath the swarf. Correct the dirt recirculation in an individual machine by having the machine dumped, cleaned, rinsed with fresh water, and charged with a fresh fluid mix at the recommended concentration. For information on Armor Protective Packaging®’s full line of corrosion management products, visit the ARMOR website or contact your ARMOR sales representative. |